HYDROPHOBIC SURFACES AND THE LOTUS EFFECT
Why do some surfaces get wet and others don't?
Hydrophilic surfaces (water absorbent) and hydrophobic (water repellent) surfaces are the result of two interactions:
- Cohesive force (CF): the attraction of liquid particles to one another. This causes a droplet to assume a spherical shape and prevent contact with surface.
- Adhesive force (AF): the attraction of liquid particles to object surfaces. This causes a droplet to spread making surfaces wet.
If CF is < AF --> the surface will get wet (hydrophilic)
If CF is > AF --> the surface will not get wet (hydrophobic)
What is Superhydrophobicity?
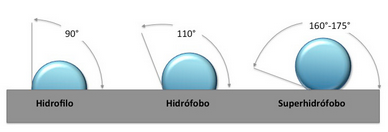
Superhydrophobic surfaces highly repel water. This property is commonly known as the Lotus Effect as the property exhibited by Lotus leafs.
Although at first sight Lotus leafs seem perfectly smooth, the leaf surface has roughness at atom scale and the air trapped in its cracks prevents water adherence to the solid. This causes the droplet to slide down the surface at the smallest surface angle. Dirt particles are collected by water droplets and this self-cleaning property protects plants against pathogens such as fungi and algae growth.
 Namazu-tron [CC BY-SA 3.0], Wikimedia Commons
Namazu-tron [CC BY-SA 3.0], Wikimedia Commons
 William Thielicke [CC BY-SA 4.0-3.0-2.5-2.0-1.], Wikimedia Commons
William Thielicke [CC BY-SA 4.0-3.0-2.5-2.0-1.], Wikimedia Commons
|
How to achieve superhydrophobic surfaces Nanotechnologists have created superhydrophobic surfaces by means of different methods:
(the easiest and least costly technique);
(the most sophisticated technology yet achieving the best results) Examples in nature Plants such as the lotus flower, water lily, cabbage, daffodils, lady's mantle, butterfly, dragonfly. Possible applications: any type of coatings for fabrics, timber, metals, ceramics, varnishes, mobile phone screens, building glazing or faster swimming costumes.
|